Gone are the days when 3D printers merely built plastic trinkets — scientists say 3D-printed structures loaded with embryonic stem cells could one day help doctors print out micro-organs for transplant patients.
Embryonic stem cells, obtained from human embryos, can develop into any kind of cell in the body, such as brain tissue, heart cells or bone. This property makes them ideal for use in regenerative medicine — repairing and replacing damaged cells, tissues and organs.
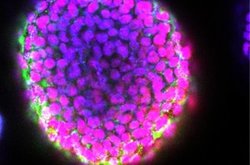
Scientists typically experiment with embryonic stem cells by dosing them with biological cues that guide them toward developing into specific tissue types — a process called differentiation. This process begins with the cells forming spherical masses called embryoid bodies — an activity that mimics the early stages of embryonic development.
Previous research suggested the best way to grow embryonic stem cells is not in flat lab dishes, but in 3D environments that mimic how these cells might develop in human bodies. Recently, scientists developed 3D printers for embryonic stem cells. A 3D printer works by depositing layers of material, just as ordinary printers lay down ink, except it can also lay down flat layers on top of one another to build 3D objects.
Until now, 3D printers for embryonic stem cells just generated flat arrays or simple mounds, called "stalagmites," of cells. Now, researchers say they have, for the first time, developed a way to print 3D structures laden with embryonic stem cells.
"We are able to apply a 3D-printing method to grow embryoid bodies in a controlled manner to produce highly uniform blocks of embryonic stem cells," study co-author Wei Sun, a professor of mechanical engineering at Tsinghua University in Beijing and Drexel University in Philadelphia,told Live Science.
In principle, these blocks could be used like Lego bricks to build tissues "and potentially even micro-organs," Sun added.
To read the entire article, click here.
 RSS Feed
RSS Feed