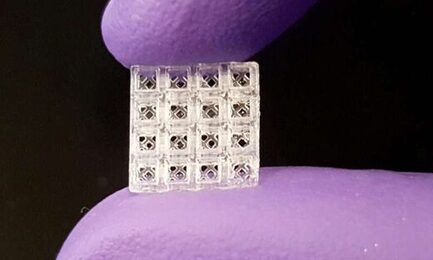
Oregon Health & Science University researchers have developed a tiny, 3D-printed technology that can be assembled like Lego blocks and help repair broken bones and soft tissue.
Inspired by Lego blocks, the small, hollow bricks serve as scaffolding onto which both hard and soft tissue can regrow better than today's standard regeneration methods, according to new research published in Advanced Materials. Each brick is 1.5 millimeters cubed, or roughly the size of a small flea.
"Our patent-pending scaffolding is easy to use; it can be stacked together like Legos and placed in thousands of different configurations to match the complexity and size of almost any situation," said Luiz Bertassoni, Ph.D., who led the technology's development and is an associate professor in the OHSU School of Dentistry and an associate professor of biomedical engineering in the OHSU School of Medicine. Bertassoni partnered with colleagues from OHSU, University of Oregon, New York University and Mahidol University in Thailand to develop and evaluate the technology.
When stacked together, the microcages are designed to repair broken bones better than today's methods. Orthopaedic surgeons typically repair more complex bone fractures by implanting metal rods or plates to stabilize the bone and then inserting bio-compatible scaffolding materials packed with powders or pastes that promote healing.
A unique advantage of this new scaffolding system is that its hollow blocks can be filled with small amounts of gel containing various growth factors that are precisely placed closest to where they are needed. The study found growth factor-filled blocks placed near repaired rat bones led to about three times more blood vessel growth than conventional scaffolding material.
"The 3-D-printed microcage technology improves healing by stimulating the right type of cells to grow in the right place, and at the right time," said study co-author Ramesh Subbiah, Ph.D., a postdoctoral scholar in Bertassoni's OHSU lab who specializes in growth factor delivery. "Different growth factors can be placed inside each block, enabling us to more precisely and quickly repair tissue."
The small devices are modular and can be assembled to fit into almost any space. When piecing together block segments containing four layers of four-bricks-by-four bricks, the researchers estimate more than 29,000 different configurations can be created.
Bertassoni and colleagues also imagine their 3-D-printed technology could be used to heal bones that have to be cut out for cancer treatment, for spinal fusion procedures and to build up weakened jaw bones ahead of a dental implant.
And, by changing the composition of the technology's 3-D-printed materials, they envision it could also be used to build or repair soft tissues. With significantly more research, they hope the modular microcage approach could even be used to make organs for transplant.
Bertassoni and his team will further explore the microcages' performance in bone repair. They plan to test the technology's ability to repair more complex bone fractures in rats or larger animals.
More information: Ramesh Subbiah et al, 3D Printing of Microgel‐Loaded Modular Microcages as Instructive Scaffolds for Tissue Engineering, Advanced Materials (2020). DOI: 10.1002/adma.202001736
 RSS Feed
RSS Feed